Weinberg, Sandy
Guidebook for Drug Regulatory Submissions
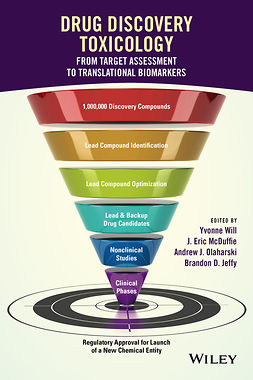
The high-stakes process of submitting drug documents and applications for regulatory review can be intimidating, particularly for the inexperienced regulatory professional charged with preparing a major regulatory submission. This book provides regulatory professionals with the key tools necessary to submit major documents to the United States Food and Drug Administration. The book consists of thirteen chapters, including an introductory and conclusion chapter and 11 units, each consisting of an introductory essay, submission checklist, evaluation checklist giving guidance on FDA criteria, and copies of relevant FDA documents.
Nyckelord: FDA, Food and Drug Administration, FDA documents, drug development, drug research, pharmaceuticals
- Författare
- Weinberg, Sandy
- Utgivare
- John Wiley and Sons, Inc.
- Utgivningsår
- 2009
- Språk
- en
- Utgåva
- 1
- Sidantal
- 352 sidor
- Kategori
- Hälsa, skönhet, mode
- Format
- E-bok
- eISBN (PDF)
- 9780470456170